EXPANDED ACCESS STATEMENT
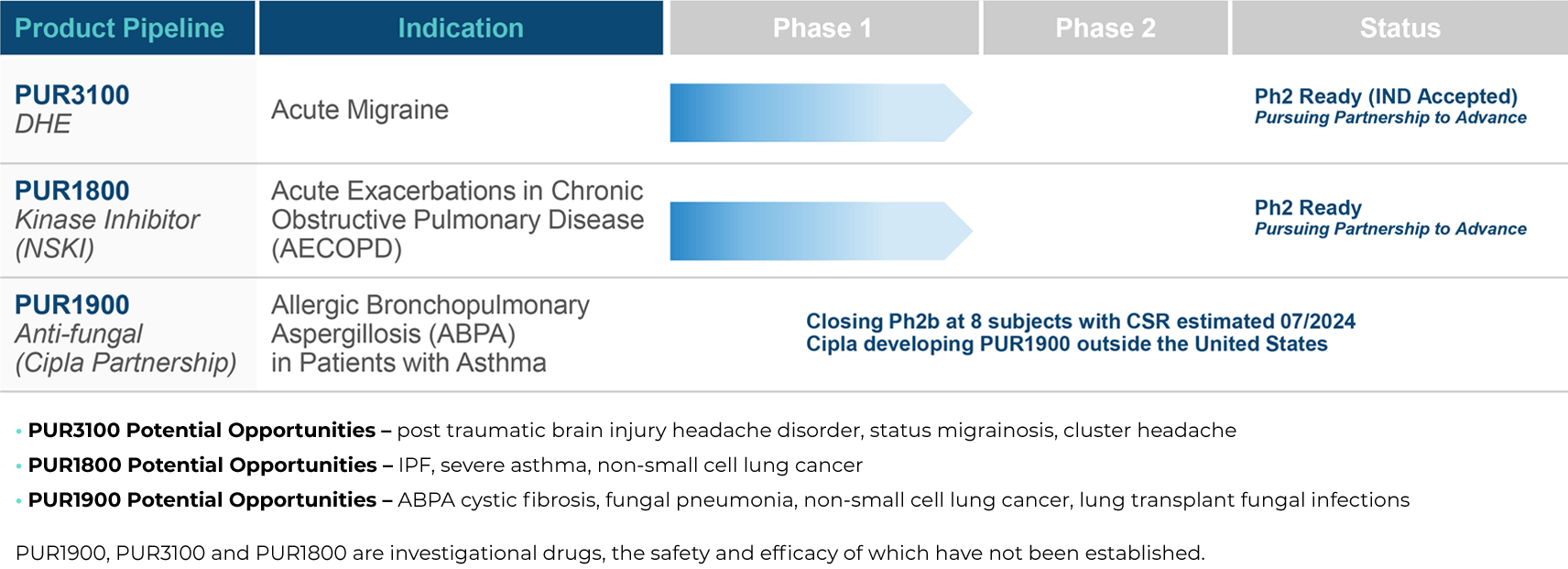
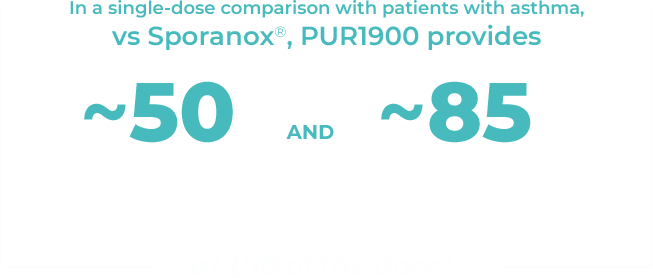
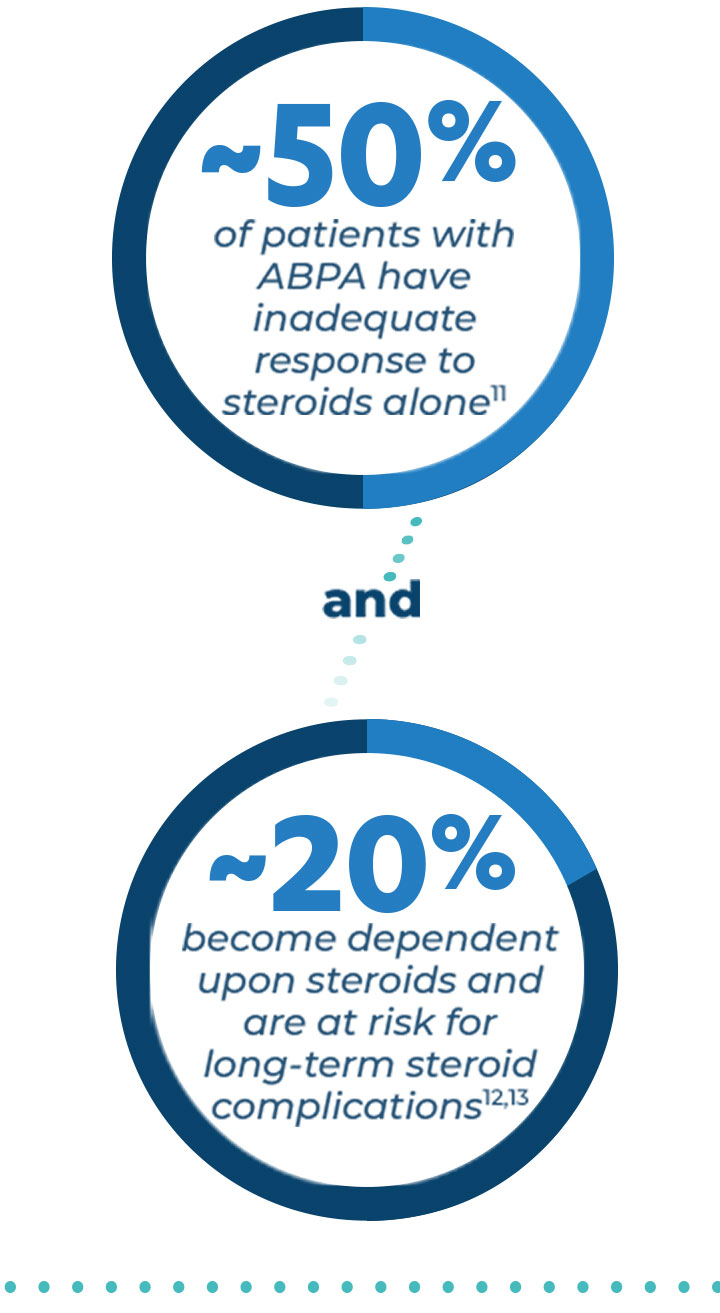
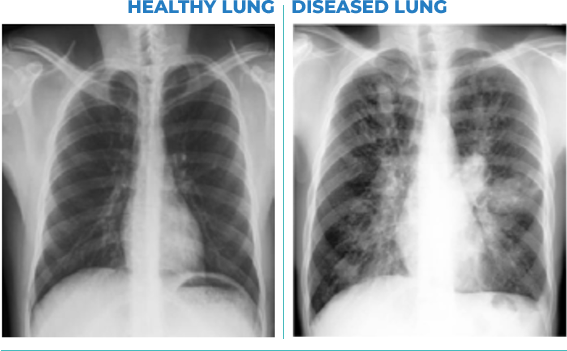
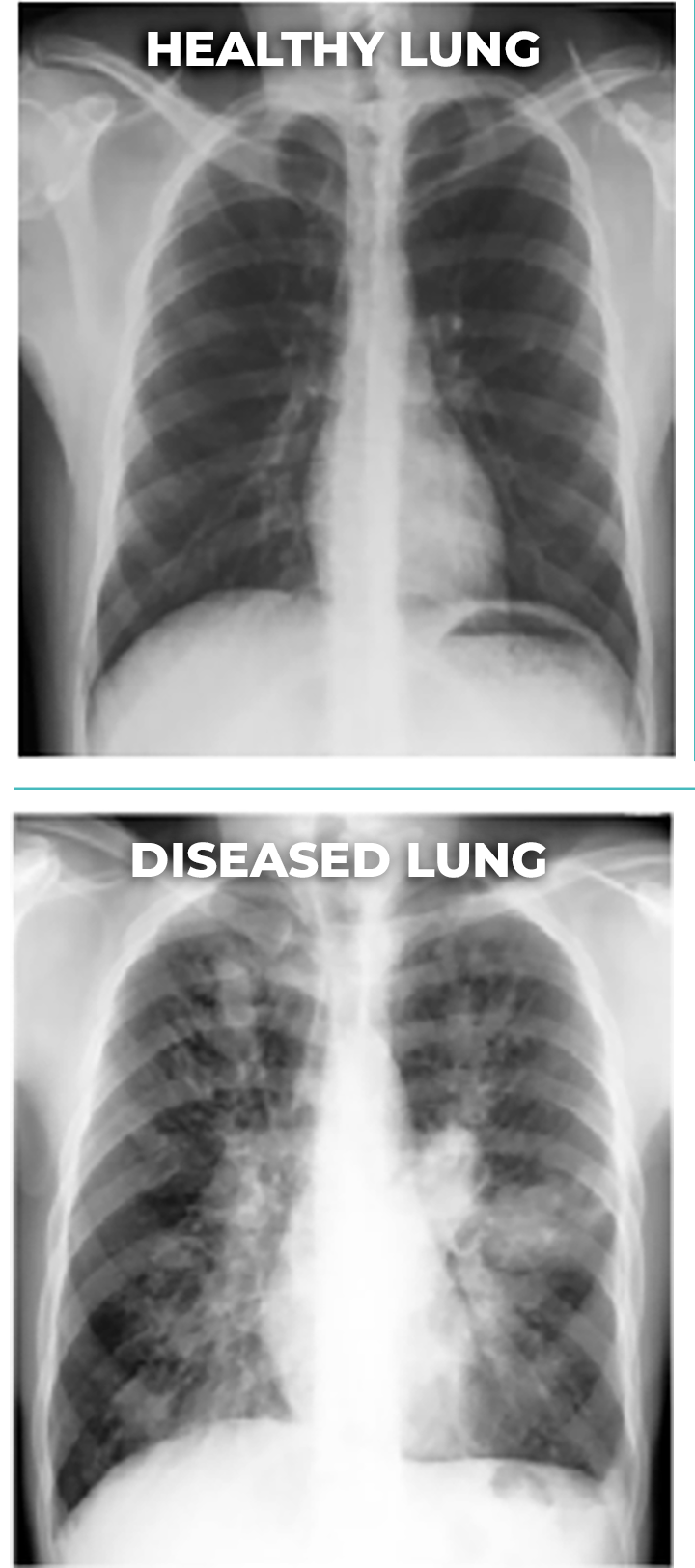
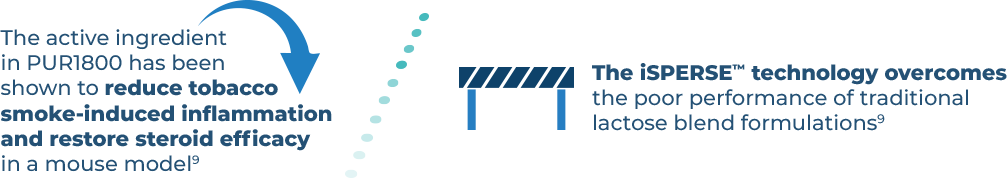
PULMATRiX is a clinical stage biopharmaceutical company developing innovative inhaled therapies to address serious pulmonary disease using its patented iSPERSE™ technology. PULMATRiX is currently developing PUR1900, an investigational product intended for the treatment of allergic bronchopulmonary aspergillosis (ABPA). Currently there is no approved therapy for ABPA, and patients with ABPA are treated with corticosteroids to counter the inflammatory response to the Aspergillus fungus that lives in the lungs of these patients. Some patients with ABPA also receive systemic antifungal therapy, such as itraconazole. Although these therapies are effective in some patients with ABPA, the use of corticosteroids and systemic antifungals is limited by the potential for serious side effects. PUR1900 is a dry powder iSPERSE™ inhaled formulation of itraconazole.
A crucial step in the development of investigational products is to conduct clinical trials. Therapeutic products being studied in clinical trials have unknown benefits and unknown risks that will not be understood until the clinical trials are complete. If results from the clinical trials are favorable, they will be submitted to FDA and other regulatory bodies for review of the drug’s safety and efficacy in order to seek approval for the product. Obtaining regulatory approval for a new medicine is the best way to bring rapid access to the greatest number of patients who may benefit.
Sometimes patients may be able to access investigational products outside of a clinical trial. In the United States, this is possible through an expanded access program, also referred to as “compassionate use.” Use of the investigational product in an expanded access program is usually separate from the development program for that product, or at best an adjunct to the carefully designed, controlled, and monitored clinical studies conducted to demonstrate safety and efficacy of the product.
It is very important to systematically obtain information about the safety and tolerability of investigational products in a controlled manner. Currently a safety and tolerability study of PUR1900 is being conducted in patients with stable asthma and ABPA. Data on safety and tolerability of PUR1900 in these patients are not yet available. In the absence of these data, PULMATRiX is not accepting expanded access requests at this time. As information from our clinical trials become available, PULMATRiX will reevaluate this policy and publish any changes to this policy if appropriate.
If you have questions about this policy, or would like information about how to enroll in PUR1900 clinical studies, please contact PULMATRiX at info@pulmatrix.com. You can also obtain information about the current study at https://clinicaltrials.gov.
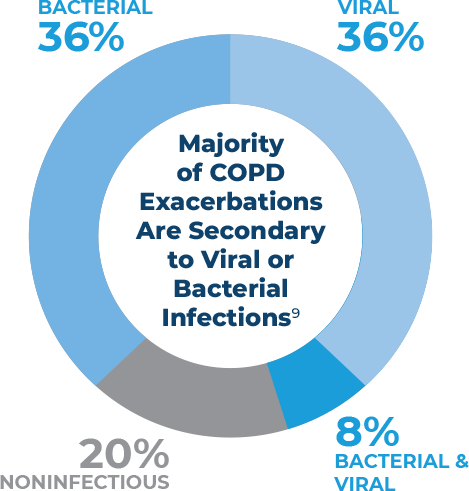
References: 1. Tepper SJ, Kori SH, Goadsby PJ. MAP004, orally inhaled dihydroergotamine for acute treatment of migraine: efficacy of early and late treatments. Mayo Clin Proc. 2011;86(10):948-955. 2. Aurora SK, Winner P, Freeman SC, et al. Onabotulinumtoxin A for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51(9):1358-1373. 3. Winner P, Ricalde O, Le Force B. A double-blind study of subcutaneous dihydroergotamine vs subcutaneous sumatriptan in the treatment of acute migraine. Arch Neurol. 1996;53(2):180-184. 4. Saper JR, Silberstein S, Dodick D, et al. DHE in the pharmacotherapy of migraine: potential for a larger role. Headache. 2006;46(suppl 4):S212-S220. 5. Aurora SK, Rozen TD, Kori SH, et al. A randomized, double-blind, placebo-controlled study of MAP0004 in adult patients with migraine. Headache. 2009;available at: https://doi.org/10.1111/j.1526-4610.2009.01453.x. 6. Migraine Research Foundation. Available at: https://migraineresearchfoundation.org. Accessed November 24, 2020. 7. Agarwal R, Dhooria S, Singh Sehgal I, et al. A randomized trial of itraconazole versus prednisolone in acute-stage ABPA complicating asthma. CHEST. 2018.doi: 10.1016/ j.chest.2018.01.005. 8. Lestner JM, Roberts SA, Moore CB, et al. Toxicodynamics of itraconazole: implications for therapeutic drug monitoring. Clin Infec Dis. 2009;49:928-930. 9. Data on file. PULMATRiX: Lexington, MA. 10. Greenberger PA, Bush RK, Demain JG, et al. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract. 2014; 2(6): 703–708. 11. Agarwal R, Aggarwal AN, Dhooria S, et al. A randomised trial of glucocorticoids in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Eur Respir J. 2016;47:385-387. 12. Shah A, Panjabi C. Allergic bronchopulmonary aspergillosis: a perplexing clinical entity. Allergy Asthma Immunol Res. 2016;8(4):282-297. 13. Patterson R, Greenberger PA, Halwig JM, et al. Allergic bronchopulmonary aspergillosis: natural history and classification of early disease by serologic and roentgenographic studies. Arch Intern Med. 1986;146(5)916-918. 14. Denning DW, Ribaud P, Milpied N, et al. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infec Dis. 2002;34:563-571. 15. Kousha M, Tadi R, and Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20(121):156-174. 16. Patterson K, Strek ME. Allergic bronchopulmonary aspergillosis. Proc Am Thorac Soc. 2010;7(3):237-244. 17. Agarwal R, Chakrabarti A, Shah A, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy. 2013;43(8):850-873. 18. Curran AK, Charron C, Russel P, et al. PUR1800 (RV1162), a novel narrow spectrum kinase inhibitor, but not fluticasone, reduces TNFα-induced cytokine release by primary bronchial epithelial cells from healthy volunteers and COPD patients. Presented at: ERS International Congress; Paris, FRA: September 2018. 19. Barnes PJ. Kinases as novel therapeutic targets in asthma and chronic obstructive pulmonary disease. Pharmacol Rev. 2016;68:788-815. 20. Geraghty P, Hardigan A, Foronjy RF. Cigarette smoke activated the proto-oncogene c-Src to promote airway inflammation and lung tissue destruction. Am J Respir Cell Mol Biol. 2013;50(3):559-570. 21. Angata T, Ishii T, Motegi T, et al. Loss of siglec-14 reduces the risk of chronic obstructive pulmonary disease exacerbation. Cell Mol Life Sci. 2013;70(17):3199-3210. 22. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128-1138. 23. Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2013;131(3):636-645. 24. Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. 25. Anzueto A. Primary care management of chronic obstructive pulmonary disease to reduce exacerbations and their consequences. Am J Med Sci. 2010;340(4):309-318. 26. Ko FW, Chan KP, Hui DS, et al. Acute exacerbation of COPD. Respirology. 2016;21:1152-1165. 27. Celli BR and Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 2007;29:1224-1238. 28. Seemungal TAR, Donaldson GC, Bhowmik A, et al. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Resp Crit Care Med. 2000;161:1608-1613.